All relevant units:
The Measures for the Rapid Review and Approval of Medical Devices in Beijing have been deliberated and adopted at the 5th Director’s Office Meeting of Beijing Food and Drug Administration in 2018, and are hereby issued, please follow them.
Beijing Food and Drug Administration
February 9, 2018
Measures of Beijing Municipality for the Examination and Approval of Rapid Review of Medical Devices
the first According to the requirements of the Notice of the General Office of the State Council City, the General Office of the Central Committee of the CPC on Printing and Distributing Opinions on Deepening the Reform of Review and Approval System and Encouraging the Innovation of Medicines and Medical Devices (Z.Z. [2017] No.42), according to the Regulations on the Supervision and Administration of Medical Devices, the Measures for the Administration of the Registration of Medical Devices, the Measures for the Administration of the Registration of In-vitro Diagnostic Reagents and the Policy Matters of the Food and Drug Administration on Supporting the Supervision and Industry Development of Zhongguancun, In order to encourage innovation, encourage the transformation of scientific and technological achievements, improve the efficiency of examination and approval of medical devices in Beijing, and promote the rapid development of medical device industry in Beijing, these measures are formulated.
the second For Beijing medical device products that meet one of the following circumstances, the applicant may apply to Beijing Food and Drug Administration for approval of innovative medical devices:
(1) Having relevant national and Beijing scientific research projects, and the patents of core technology inventions involved have been made public or authorized.
(2) The invention patent of the core technology, which is the first in Beijing, has the leading product technology in China, has great clinical application value and is involved, has been made public or authorized.
(3) Patents for core technology inventions produced by key enterprises in Beijing, such as the 100-thousand-cultivation project and the Beijing Biomedical Industry Leap-forward Development Project (G20), have been made public or authorized.
(four) included in the national or Beijing major science and technology projects, key research and development plans;
Article For the implementation of innovative medical devices in accordance with the provisions of Article 2 of these Measures, the person in charge shall be responsible, and early intervention, whole-process counseling, priority registration and testing, priority review and approval, priority registration quality system verification and priority production license matters shall be handled. See Annex 1 for the application requirements of innovative medical devices in Beijing.
Article 4 According to the application of the enterprise, Beijing Medical Device Technology Evaluation Center provides pre-consulting services for innovative clinical trial schemes of medical devices that meet the requirements of Article 2 of these Measures.
Article 5 For innovative medical devices that meet the requirements of Article 2 of these Measures, enterprises may entrust Beijing medical device manufacturing enterprises to produce products, and the medical device production license of the entrusted party shall obtain the corresponding production scope.
Article 6 For innovative medical devices, a cross-disciplinary joint evaluation method can be implemented.
Article 7 For innovative medical devices in Beijing that have been listed in the first registration cycle, all adverse medical device events of the product shall be reported and a summary report on monitoring, analysis and evaluation of adverse medical device events shall be submitted to the Beijing medical device adverse event monitoring technical institution and the food and drug administration department in the jurisdiction every year.
Article 8 For Beijing medical device products that meet one of the following circumstances, the applicant may apply to Beijing Food and Drug Administration for priority approval of medical devices:
(1) It is urgently needed in clinic, and there is no product of the same variety approved for registration in China;
(two) the varieties of medical devices belonging to the Beijing medical materials reserve unit and the unique and frequently-occurring diseases of children or disabled people;
(3) Diagnosing or treating rare diseases with obvious clinical advantages;
(four) for the treatment of serious life-threatening diseases and there is no effective treatment, as well as public health and other urgent needs;
(5) Intelligent rehabilitation apparatus;
(six) Beijing innovative medical device products to improve the production process.
Article 9 For medical device products that meet the requirements of Article 8 of these Measures, priority shall be given to registration and testing, priority shall be given to review and approval, priority shall be given to the verification of registration quality system, and priority shall be given to the handling of production licensing matters. Please refer to Annex 2 for the application requirements for priority approval of medical devices in Beijing.
Article 10 For the change of medical device registration license matters, the specification of product name, product technical requirements, scope of application, etc. does not involve substantial content changes, which can be combined with the renewal of registration.
Article 11 If the recommended standards, the guiding principles of registration technology review and the norms of registration technology review change in the renewal registration of medical devices, the enterprise may not change the licensing matters.
Article 12 If the specifications and models of medical devices are reduced, they can be handled in accordance with the registration procedures.
Article 13 For in vitro diagnostic reagent products, it is not necessary to submit analytical performance evaluation data, risk analysis data related to product changes, product technical requirements, product specifications and label samples by increasing the packaging specifications with different loading quantities (only the loading differences) and adding the changes of licensing items for applicable models with the same degree of automation. The time limit for registration review was shortened to 30 working days.
On the basis of strictly implementing the requirements of the quality system, the enterprise completes relevant risk analysis, performance evaluation, design changes, etc., and keeps records for future inspection.
Article 14 Those who fail to apply for registration renewal six months before the expiration of the validity period shall be registered for the first time. If the product has not changed, the latest registered clinical data, registration test report and system verification results may be submitted.
Article 15 Formulate the technical guidelines for clinical evaluation of medical devices in Beijing, study the comparison methods of similar medical devices, and simplify the clinical evaluation data of the same variety of medical devices.
Article 16 Beijing Medical Device Technology Evaluation Center has established a communication mechanism for enterprises to solve the difficult problems encountered by enterprises in the registration and evaluation stage of medical devices.
Article 17 Integrate the on-site inspection of the registered quality system verification and the on-site inspection of the production license. In principle, after the on-site inspection of the new product registration quality system verification has passed and the medical device registration certificate has been obtained, the products added to the Medical Device Production License can be exempted from the on-site inspection.
Article 18 For enterprises that have obtained production licenses, applying for the registration of Class II medical devices that do not involve new methodology or new technology may be exempted from on-site inspection or optimization of on-site inspection items and processes in the process of registration quality system verification.
Article 19 If the contents of the change of medical device registration license items do not involve the change of production process, the on-site inspection may be exempted or the on-site inspection items and processes may be optimized in the process of registration quality system verification.
Article 20 For those who have passed the on-site inspection of registered quality system verification at least once in two years, and the products applied for inspection this time have the same or similar working principles and expected uses compared with those that have passed the inspection, and have basically the same structural composition, production conditions and production processes, they are exempted from on-site inspection or optimized on-site inspection items and processes in the process of registered quality system verification.
Article 21 If the production address is reduced and the production process changes are not involved, the on-site inspection can be exempted or the on-site inspection items and processes can be optimized during the approval of the production license.
Article 22 In principle, on-site re-inspection is not required if no key project defects are found in the on-site inspection of the registered quality system, and the number of general project defects accounts for less than 10% of the total number of general projects that should be inspected.
Article 23 If the on-site inspection of the production license requires the enterprise to carry out rectification, the enterprise may be required to submit rectification report and rectification data according to the situation of the on-site inspection and the rectification items, and the on-site re-inspection is not required if it can be verified through the data.
Article 24 Optimize the registration examination and approval procedures, and cancel the following examination and review links in the administrative examination and approval of medical device registration:
(1) First registration of Class II medical device products (including in-vitro diagnostic reagents) that fully implement the guiding principles of product registration technology review issued by china food and drug administration or the product registration technology review norms issued by Beijing Food and Drug Administration;
(two) the second class of medical devices (including in vitro diagnostic reagents) continued registration;
(three) the second kind of medical devices (excluding in vitro diagnostic reagents) product description changes;
(four) the second kind of medical devices (including in vitro diagnostic reagents) product registration certificate error correction.
Article 25 These Measures shall not apply to domestic applicants who apply for approval of innovative medical devices to Beijing Food and Drug Administration according to the Notice of the General Administration of Food and Drug Administration on Printing and Distributing Special Approval Procedures for Innovative Medical Devices (Trial) (No.13 [2014] of the Food and Drug Administration).
Article 26 These Measures shall be interpreted by the Beijing Food and Drug Administration.
Article 27 These Measures shall come into force as of the date of promulgation. The Measures for the Examination and Approval of Medical Devices in Beijing (Trial) issued on August 11, 2016 (Beijing Food and Drug Administration [2016] No.36) was abolished at the same time.
Attachment: 1. Requirements for reporting innovative medical devices in Beijing.
2. Requirements for priority examination and approval of medical devices in Beijing
Annex 1
Requirements for reporting innovative medical devices in Beijing
In order to standardize the application for innovative medical devices in Beijing and improve the quality of application materials, this application requirement is formulated in accordance with the Measures for the Examination and Approval of Rapid Medical Devices in Beijing.
I. Application materials for innovative medical devices in Beijing
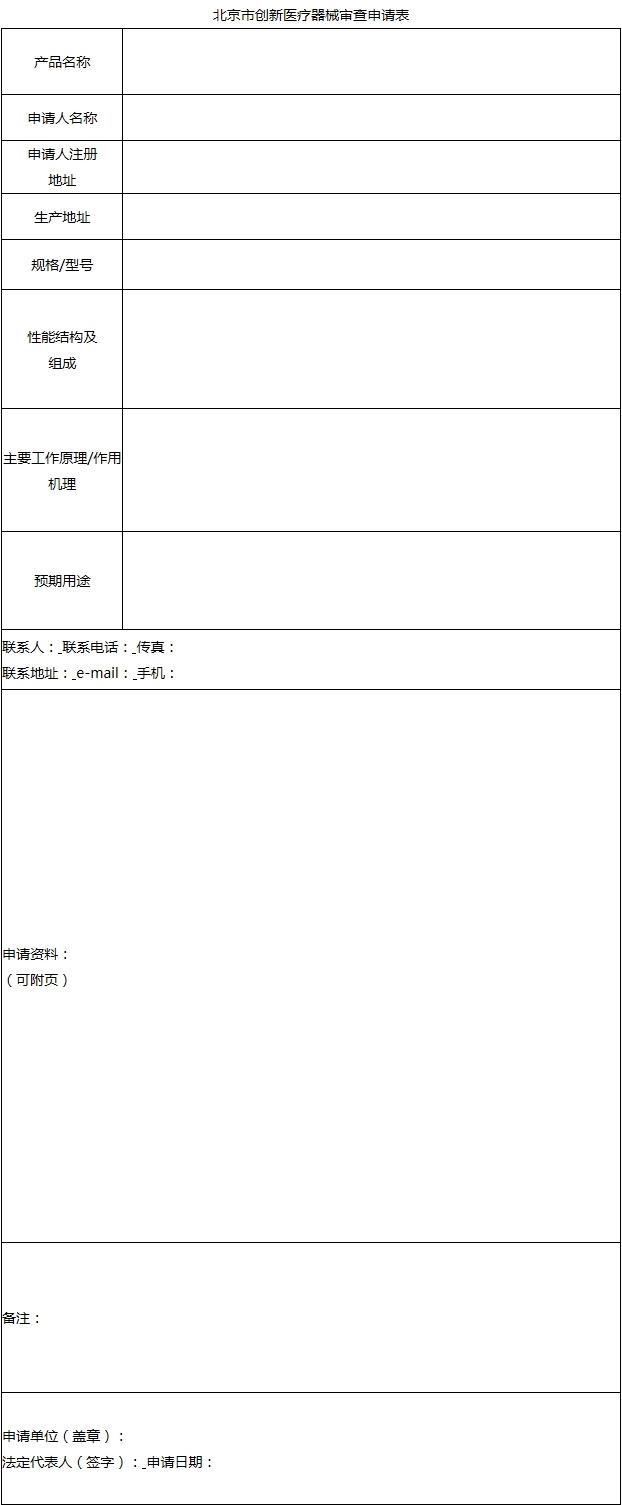
(a) Beijing innovative medical device review application form (Annex 1)
The applicant shall truthfully fill in all the contents.
The contents filled in the product performance structure and composition, main working principle/action mechanism and expected use should reflect all the important information of product characteristics, be concise and to the point, use standardized and professional terms, and avoid ambiguity.
(2) Relevant information of previous applications for innovative medical device approval (approval) (if applicable)
If the product has applied for innovative medical device products from china food and drug administration or Beijing Food and Drug Administration, for the innovative medical devices that have been applied for again, copies of previous notices shall be provided, and explanations on product changes and improvement of application materials shall be submitted.
(3) A photocopy of the business license of the enterprise to which it applies.
(four) the intellectual property rights of the products and the supporting documents.
1. Provide a description of the intellectual property rights of the core technology of the innovative medical device applied for. If there are many invention patents, it is suggested to display the information such as the name of the invention patent, the patentee and the patent status in a list.
2. Provide relevant intellectual property documents.
(1) If the applicant has obtained the patent right for invention, it is required to provide the original copy of the patent power of attorney, the patent claim, the specification and the copy of the patent register signed by the applicant.
(2) If the applicant has obtained the right to use the invention patent in China through the transferee according to law, in addition to submitting the copy of the patent authorization, patent claim, specification and patent register held by the patentee, it is also necessary to provide the original Certificate of Filing the Patent License Contract issued by the patent administrative department.
(3) If the application for a patent for invention has been made public by the patent administration department of the State Council and is not authorized, it is necessary to provide a copy of the proof document of the publication of the invention patent signed by the applicant (such as the notice of publication of the application for a patent for invention, the notice of publication of the application for a patent for invention and the notice of entry into the substantive examination stage, etc.) and a copy of the published version of the claim and specification. In the process of examining an application for a patent for invention, if the patent claim and specification are modified at the request of the patent examination department, the modified text shall be submitted; Where the patentee changes, the certifying documents issued by the competent patent department, such as a copy of the notice of conformity, shall be submitted.
(V) Summary of product development process and results
Summarize the original intention of product research and development, laboratory research, animal experimental research, clinical research and results, and submit a comprehensive report on product research and development including design input, design verification and design output. Information on patient selection criteria, as well as parameters to be monitored and factors to be considered during use.
(6) Product technical documents, which shall at least include:
1. The intended use of the product;
(1) It should be made clear that the treatment and diagnosis expected to be provided by the product conform to the purpose defined in Article 76 of the Regulations on the Supervision and Administration of Medical Devices, and the applicable medical stage (such as monitoring and rehabilitation after treatment) can be described;
(2) explain whether the product is used once or repeatedly;
(3) A description of the equipment expected to be used in combination with it;
(4) Information of the target patient population (such as adults, children or newborns).
2. Working principle/action mechanism of the product;
Elaborate the working principle/mechanism of the product to achieve its expected use, and provide relevant basic research data.
3. Make clear the main technical indexes of products and the basis for determination, the index requirements of main raw materials and key components, the main production process and flow chart, and the inspection methods of main technical indexes.
(seven) certification documents of product innovation, which shall at least include:
1. Novelty retrieval report issued by information or patent retrieval institutions.
It should be a sci-tech novelty report issued by information retrieval institutions in China or a novelty report issued by patent retrieval institutions. The contents of the report should be able to prove the innovation point, innovation level and reasons of the product. The novelty retrieval report is valid for one year.
2. Summary of academic papers, monographs and documents published in core journals that can fully explain the clinical application value of products (if any)
Can provide product literature, but also provide overseas literature of similar products.
3. Analysis and comparison of the application of similar products listed at home and abroad.
(1) Provide a description of the retrieval of similar products that have been listed in China. Generally, it should include the retrieval database, retrieval date, retrieval keywords and the results retrieved by each keyword, and analyze the differences between the applied innovative medical devices and similar products on the market in terms of working principle/mechanism.
(2) Provide an explanation of the application of similar products listed overseas, compare and analyze the similarities and differences with this product, and provide comparative analysis data to support this product to be at the international leading level in technology.
4. The innovative content of the product and its remarkable value in clinical application.
(1) Overview of innovative medical devices applied for.
This paper expounds the innovative contents of the applied medical device, and discusses that the device has been significantly improved in terms of safety, effectiveness and economy compared with the existing products or treatment methods through innovation, and has significant clinical application value.
(2) Provide relevant supporting materials.
(eight) product safety risk management report.
1. Based on the research results of the risk management process carried out by the product.
2. Prepared according to YY/T 0316 "Application of Medical Device Risk Management to Medical Devices".
(9) Product description (sample draft).
It shall comply with the relevant requirements in the Regulations on the Administration of Instructions and Labels of Medical Devices (Order No.6 of the General Administration).
(ten) other documents that prove that the product conforms to Article 2 of the Measures for the Examination and Approval of Medical Devices in Beijing.
1. If the product or its core technology has won national and Beijing municipal science and technology awards, please explain and submit copies of relevant supporting documents;
2. The approval certificate of key supporting enterprises in Beijing, such as the 100 thousand cultivation project and the Beijing biomedical industry leap-forward development project (G20).
3 included in the national or Beijing major science and technology projects, key research and development plan documents.
(eleven) the self-assurance statement of the authenticity of the submitted materials.
Second, the format requirements
(a) the application materials shall be compiled by the applicant, and the documents shall be printed on A4 paper, arranged in the order specified in this acceptance requirement and bound into a book.
(two) there should be a list of submitted materials, including the first and second titles of the application materials. The data corresponding to each secondary heading shall be separately numbered.
(3) Unless otherwise specified, the application materials for the review of innovative medical devices in Beijing shall be original and signed by the applicant. "Signature" refers to the seal of the enterprise, or the signature of its legal representative and responsible person plus the seal of the enterprise.
(four) if the application materials are in photocopy, the photocopy shall be clear and consistent with the original. Color pictures and charts shall be provided with color copies. If the original application materials have been submitted in the previous application for examination of innovative medical devices, a copy signed by the applicant can be provided, and the applicant will issue a document stating the acceptance number of the application materials where the original application materials are located.
(5) The application materials shall be in Chinese. If the original text is in a foreign language, there should be a Chinese translation.
Third, the review process
To apply for innovative medical device products in our city, after the above materials are prepared, they shall be reported to the Medical Device Registration and Supervision Department of the Municipal Bureau (hereinafter referred to as the "Device Department"), and the Device Department shall formally review the application materials, and issue a receipt document (Annex 2) within 5 working days; The device will be reviewed by relevant experts within 40 working days, and the applicant and product name will be publicized on the website of Beijing Food and Drug Administration for 10 working days for the varieties to be included in Beijing’s innovative medical devices. If there is no objection after the publicity period, a final review opinion will be issued, and a notice of review of innovative medical devices in Beijing (Annex 3) will be made to inform the applicant in writing.
Attachment: 1. Application Form for Review of Innovative Medical Devices in Beijing
2. Beijing Innovative Medical Devices Review Receipt Certificate
3. Notice of Review Opinions on Innovative Medical Devices in Beijing
Annex 1 of Beijing Municipality’s Declaration Requirements for Innovative Medical Devices
Annex 2 of Beijing Municipality’s Declaration Requirements for Innovative Medical Devices
Beijing Innovative Medical Device Review Receiving Material Voucher
(first copy)
(Receiving material number: _ _ _ _ _)
:
Your company applied for the examination of innovative medical devices in Beijing. After formal examination, the application materials are complete and it is decided to accept it. A total of _ _ pieces of materials were received.
Tel: 010-83979525
Receiver’s signature: year month day
Beijing Innovative Medical Device Review Receiving Material Voucher
(second copy)
(Receiving material number: _ _ _ _ _)
:
Your company applied for the examination of innovative medical devices in Beijing. After formal examination, the application materials are complete and it is decided to accept it. A total of _ _ pieces of materials were received.
Tel: 010-83979525
Signature of the applicant: year month day
Annex 3 of Beijing Municipality’s Declaration Requirements for Innovative Medical Devices
Notice of Beijing Municipality on Review Opinions of Innovative Medical Devices
(No.:_ _ _ _ _)
The application for examination of innovative medical devices in Beijing submitted by your company (receiving material number:)
Performance structure and composition:
Main working principle/mechanism:
The conclusion of the review is:
.
I hereby inform you.
(Seal)
Date:
Annex 2
Requirements for Priority Approval and Declaration of Medical Devices in Beijing
In order to standardize the application for priority approval of medical devices and improve the quality of application materials, this application requirement is formulated in accordance with the Measures of Beijing Municipality for Rapid Review and Approval of Medical Devices (Revised).
First, Beijing gives priority to the examination and approval of medical device application materials.
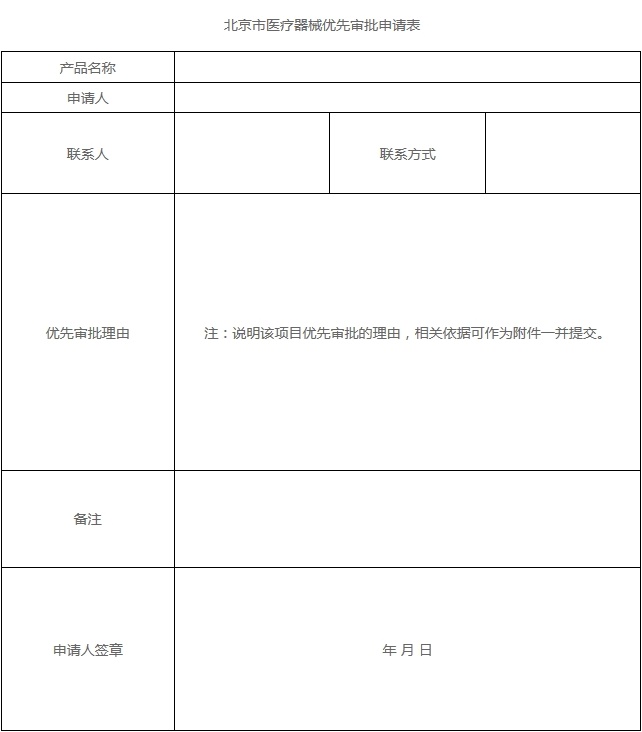
(1) Application Form for Priority Approval of Medical Devices (Annex 1)
Clearly explain the circumstances specified in Article 14 of the Measures for the Rapid Review and Approval of Medical Devices in Beijing (Revised), and briefly describe the reasons for priority approval.
(two) a copy of the application form for registration of medical devices.
(three) in accordance with the "Beijing medical device rapid review and approval measures" eighth cases of medical device priority approval application, should provide information according to the following requirements:
1 clinical needs, and there is no product of the same variety approved for registration in China.
(1) Summary of the clinical treatment status of the indications of this product, explaining the reasons for urgent clinical need;
(2) The overseas approval and clinical use of this product and similar products;
(3) Provide a description of the search, which proves that there are no related products of the same variety approved for registration in China at present, and there is no equivalent alternative diagnosis or treatment method at present.
2. The medical device reserve varieties belonging to the Beijing medical material reserve unit and the medical device varieties used by children or disabled people with unique and multiple diseases.
(1) the certificate of entering the catalogue of medical material reserve units in Beijing;
(2) The indication of this product belongs to the supporting data of unique and frequently-occurring diseases of children or disabled people;
(3) Summary of the clinical treatment status of this indication;
(4) Description and relevant supporting materials proving that the product is specially used for diagnosing or treating unique and frequently-occurring diseases of children or disabled people, and has obvious clinical advantages compared with existing products or treatment methods.
3 diagnosis or treatment of rare diseases, and has obvious clinical advantages.
(1) Incidence data of the indications of this product and relevant supporting data;
(2) Supporting data to prove that the indication is a rare disease;
(3) Summary of the clinical treatment status of this indication;
(4) Description of the obvious clinical advantages of this product compared with the existing products or treatment methods and relevant supporting materials.
4 for the treatment of serious life-threatening diseases and there is no effective treatment, as well as public health and other urgent needs.
(1) To prove that the indication belongs to the urgently needed supporting information for treating diseases that are seriously life-threatening and have no effective treatment methods, and public health;
(2) Summary of the clinical treatment status of this indication;
(3) the product has obvious clinical advantages over the existing products or treatment methods, and relevant supporting materials.
5. Intelligent rehabilitation apparatus
(1) Medical devices that meet the definition of intelligent rehabilitation devices in the "Thirteenth Five-Year Plan for Scientific and Technological Innovation of Health Industry" (No.149 [2017] of National Science and Technology Development Agency);
(2) Summary of the clinical treatment status of this indication;
(3) the product has obvious clinical advantages over the existing products or treatment methods, and relevant supporting materials.
6. Improve the production process of innovative medical device products in Beijing.
(1) Notice of Review Opinions on Innovative Medical Devices in Beijing;
(2) Description of production process changes of new and old products.
(4) A self-assurance statement on the authenticity of the submitted materials.
Second, the format requirements
(a) the application materials shall be compiled by the applicant, and the documents shall be printed on A4 paper, arranged in the order specified in this acceptance requirement and bound into a book.
(two) there should be a list of submitted materials, including the first and second titles of the application materials. The data corresponding to each secondary heading shall be separately numbered.
(3) Unless otherwise specified, the application materials for the examination and approval of priority medical devices in Beijing shall be original and signed by the applicant. "Signature" refers to the seal of the enterprise, or the signature of its legal representative and responsible person plus the seal of the enterprise.
(four) if the application materials are in photocopy, the photocopy shall be clear and consistent with the original. Color pictures and charts shall be provided with color copies. If the original application materials have been submitted in the previous priority examination and approval of the medical device application, a copy signed by the applicant can be provided, and the applicant will issue a document stating the acceptance number of the application materials where the original application materials are located.
(5) The application materials shall be in Chinese. If the original text is in a foreign language, there should be a Chinese translation.
Third, the review process
To apply for the priority approval of medical device products in our city, after the above materials are prepared, they shall be reported to the Medical Device Registration and Supervision Department of the Municipal Bureau (hereinafter referred to as the "Device Department"), and the Device Department shall conduct a formal review of the application materials, and issue a receipt document (Annex 2) within 5 working days; The device shall be reviewed by relevant experts within 40 working days, and the applicant and product name shall be publicized on the website of Beijing Food and Drug Administration for 10 working days for the varieties of medical devices to be approved in Beijing. If there is no objection after the publicity period, a final review opinion will be issued, and a notice (Annex 3) for the review of medical devices with priority approval in Beijing will be made, and the applicant will be informed in writing.
Attachment: 1. Application Form for Priority Approval of Medical Devices in Beijing
2. Beijing Municipality gives priority to the examination and approval of medical devices, and receives the certificate of materials.
3. Notice of Beijing Municipality on Priority Examination and Approval of Medical Devices
Annex 1 of the Declaration Requirements for Priority Approval of Medical Devices in Beijing
Annex 2 of the Declaration Requirements for Priority Approval of Medical Devices in Beijing
Beijing Municipality gives priority to the examination and approval of medical devices.
(first copy)
(Receiving material number: _ _ _ _ _)
:
Your company applied for the examination of medical devices with priority approval in Beijing. After formal examination, the application materials are complete and it is decided to accept it. Total materials received Pieces.
Tel: 010-83979525
Receiver’s signature: year month day
Beijing Municipality gives priority to the examination and approval of medical devices.
(second copy)
(Receiving material number: _ _ _ _ _)
:
Your company applied for the examination of medical devices with priority approval in Beijing. After formal examination, the application materials are complete and it is decided to accept it. Total materials received Pieces.
Tel: 010-83979525
Signature of the applicant: year month day
Annex 3 of the Declaration Requirements for Priority Approval of Medical Devices in Beijing
Notice of Beijing Municipality on Priority Examination and Approval of Medical Devices
(No.:_ _ _ _ _)
Your application for the examination of medical devices in Beijing with priority approval (receiving material number:)
Performance structure and composition:
Main working principle/mechanism:
The conclusion of the review is:
.
I hereby inform you.
(Seal)
Date: